Progression of Basic Research on Chalcogen Elements into Applied Research
Professor, Division of Material Science, Graduate School of Science and Engineering
|
March 1982 |
Graduation from Department of Chemistry, Faculty of Science, Saitama University |
|---|---|
|
March 1984 |
Completion of the Master Course, Graduate School of Science, the University of Tokyo |
|
March 1987 |
Completion of the Doctorate Course, Graduate School of Science, the University of Tokyo (PhD in Science) |
|
April 1987 |
Assistant Professor[A2] , Department of Chemistry, Faculty of Science, Saitama University |
|
June 1994 |
Associate Professor, Department of Chemistry, Faculty of Science, Saitama University |
|
April 2004 |
Professor, Graduate School of Science and Engineering, Saitama University |
|
1996 |
Incentive Award in Synthetic Organic Chemistry, Japan (The Society of Synthetic Organic Chemistry, Japan)[A3] |
|
1997 |
Visiting Professor, Université de Caen, France |
Plastics such as polyethylene, polypropylene and polystyrene are examples of materials indispensable for the daily living in the modern age. These materials are yielded from polymerization of alkenes such as ethylene, propylene and styrene. Their polymerization requires catalysts. Color displays are an example of product having achieved remarkable technological advances in recent years. The technology in this field is constantly advancing, yielding diverse products ranging from small-sized color displays used for cell phones, etc., to large-sized color displays (exceeding 50 inches in size). Needless to say, development of more efficient catalysts and light-emitting materials is indispensable for achieving technological innovation in these fields.
During my research at the end of my undergraduate course at the university, I dealt with sulfur compounds. Since then, including the graduate student period, I have been primarily engaged in basic research on organic compounds including chalcogen elements (elements of the oxygen group, i.e., group 16 of the periodic table) such as selenium. Sulfur and selenium are present in the nature, assuming diverse forms. They are elements playing important roles in biological activities. For example, sulfur is an element constituting cysteine and methionine (essential amino acids), and selenium is also one of the indispensable elements for organisms and is involved in the form of selenocysteine in the enzymatic reactions for oxidation and reduction.
To date, we have succeeded for the first time in the world in synthesizing/isolating compounds such as dithiirane, vic-disulfoxide, and selenoseleninic acid ester. New compounds possessing new skeletons or new forms of binding are likely to undergo new reactions, occasionally enabling discovery of new materials or reactions. During our research on dithiirane and vic-disulfoxide, we found unprecedented reactions. Making use of these reactions, we synthesized several new compounds. One of them is trans 1,2-cyclooctanedithiol. In one of the subsequent applied studies, we synthesized ligand-metal complexes with the use of this compound. The thus synthesized complexes were found to have catalytic activity much higher than existing compounds of the same category when used for alkene polymerization. Furthermore, during research on reactions of selenoseleninic acid ester with metal complexes, we discovered compounds showing fluorescence or phosphorescence with high efficiency. We are thus continuing research in two segments closely connected to each other (basic research and applied research advanced therefrom).

The outcome of our efforts to develop alkene polymerization catalysts with high activity and stereospecificity was published as a rapid communication in the Journal of the American Chemical Society
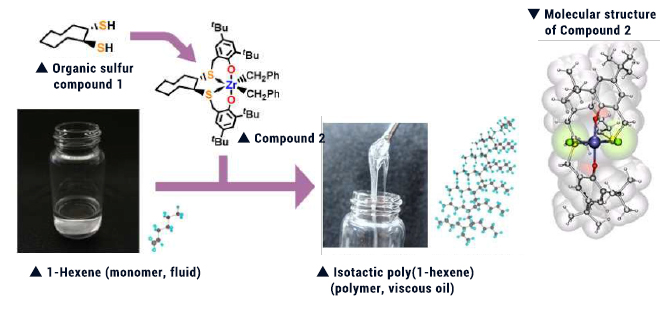
With our original organic sulfur compound 1 serving as a starting material, we designed a compound characterized by the cyclooctane ring and having two sulfur atoms and 2 oxygen atoms. Zirconium complex (Compound 2) was subsequently synthesized from this compound. Compound 2 was shown to serve as an ultra-high speed polymerization catalysts (capable of catalyzing polymerization of 1-hexene at a rate of 8.3 times/sec). The thus yielded polymer was isotactic poly(1-hexene) with side chains arranged regularly in the same direction relative to the main chain. Ligation of sulfur to zirconium has been suggested to play an important role in this complex.
This research outcome was published as a rapid communication in the Journal of the American Chemical Society, with its picture adopted on its front page, thus giving large impact not only in Japan but also world-wide.
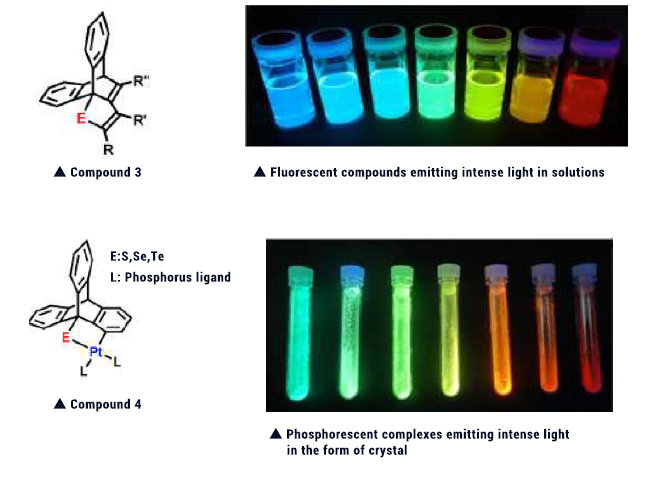
 Sulfur and selenium compounds having a special substituent were reacted with platinum complex. The resulting complex was reacted with alkynes. The thus formed Compound 3 was found to emit intense light in solutions. This finding triggered our development of simpler synthesis methods, allowing synthesis of various derivatives. Replacement of the substituent (R) of Compound 3 with another substituent allowed the color and intensity of emitted light (photoluminescent quantum yield) to be adjusted, enabling us to achieve synthesis of derivatives emitting diverse lights (from blue to red) and having up to 100% photoluminescent quantum yield. If various substituents tailored to the objectives are introduced, this compound is expected to be applicable to various uses such as chemical sensors and biolabeling materials.
Sulfur and selenium compounds having a special substituent were reacted with platinum complex. The resulting complex was reacted with alkynes. The thus formed Compound 3 was found to emit intense light in solutions. This finding triggered our development of simpler synthesis methods, allowing synthesis of various derivatives. Replacement of the substituent (R) of Compound 3 with another substituent allowed the color and intensity of emitted light (photoluminescent quantum yield) to be adjusted, enabling us to achieve synthesis of derivatives emitting diverse lights (from blue to red) and having up to 100% photoluminescent quantum yield. If various substituents tailored to the objectives are introduced, this compound is expected to be applicable to various uses such as chemical sensors and biolabeling materials.

Experimental device (glove box)
We have also synthesized platinum complex 4 having a similar skeleton. This compound was found to show diverse and intense phosphorescence in the form of crystal at room temperature. Phosphorescent compounds usually emit light at low temperature in deoxygenated dilute solutions. Complex 4 is unique in that it emits intense light in the form of a solid at room temperature. When light emission is attempted by means of electroluminescence (EL), phosphorescent compounds are capable of emitting light with higher efficiency than fluorescent compounds. So, this complex is expected to be utilized from now on as a light-emitting layer of organic EL devices.

Experimental device (vacuum line)
Compounds 3 and 4 can be both characterized by containing selenium and tellurium which have been seldom used for light-emitting organic compounds. We are conducting research day after another while dreaming development of compounds with novel functions making use of the characteristics of these elements.
We often handle unstable compounds which can be degraded immediately if exposed to ambient air. At such occasions, we make full use of experimental devices such as vacuum line and globe box to enable experiments to be carried out in the presence of inactive gas (argon gas).


© Copyright Saitama University, All Rights Reserved.